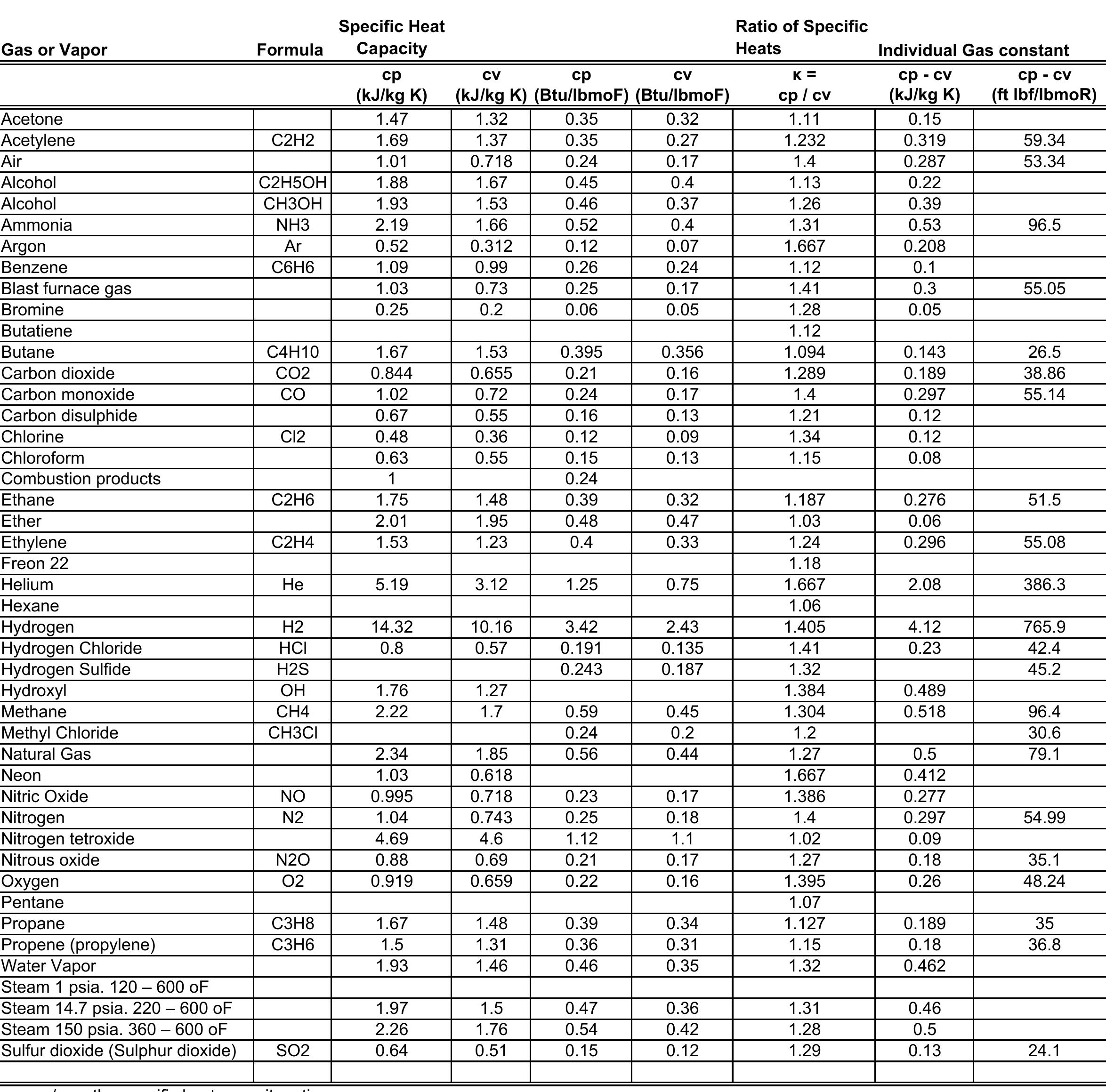
Specific heat capacity refers to the amount of heat energy needed to raise the temperature of 1 gram of a substance by 1 degree Celsius (or 1 pound by 1 degree Fahrenheit). The specific heat capacity of water is 1 Btu/lb°F. This means it takes 1 British Thermal Unit (Btu) of heat energy to raise the temperature of 1 pound of water by 1 degree Fahrenheit. The lower the number the easier it is for the material to move heat. It is one of the aspects of aluminum cans that makes cooling the beverage faster. Aluminum has a really low specific heat capacity. Lower numbers move heat better. This is why nitrogen removes heat better than hydrogen.